
Thursday, February 28, 2013
Wednesday, February 27, 2013
Balantidium coli
Balantidium coli
The ciliate protozoa form a large group of organisms that is characterised by numerous cilia. A large number of ciliates are free-living and an equally large number consists of parasites of the digestive systems of termites, cockroaches and herbivores.
A typical ciliate has a conical mouth, the cytostome, near the anterior end and an anal opening, the cytopyge, at the posterior end. Ciliates have two types of nuclei: a large kidney-shaped macronucleus and a small spherical micronucleus, both of which are visible in the cyst and in the trophozoite.

Balantidium coli is the only ciliate that is parasitic to humans. Balantidium coli has a global distribution in pigs, dogs and several species of monkeys. In man, it occurs mainly in warm climates. The parasite occurs in the large intestine of man, pigs and monkeys.
Morphology
The parasite exists as a cyst and as a trophozoite, the trophozoite being the active form. It is ovoid and covered by cilia that are perpetually in motion, which are used for movement. The parasite varies in size, ranging from 50 to 100 µm in length by 40 to 70 µm in width. The anterior end is conical and the posterior end is rounded. To one side of the anterior end, there is funnel-like peristome, which leads to the cytostome or mouth.

Balantidium coli trophozoite (left) and cyst (right)
Life cycle
Balantidium coli lives in the large intestine where it feeds on bacteria, cells and food particles present in the intestinal lumen. Asexual reproduction is by binary fission in which the micronucleus first divides followed by the division of the macronucleus, resulting in two daughter organisms. B. coli can also reproduce sexually by a process called conjugation, which involves the exchange of nuclear material.

Life cycle of B. coli. A, ingestion of cyst; B, trophozoite formed in the colon divides by binary fission;
C, trophozoite in the lumen of the large intestine or in faeces freshly voided.
Pathogenesis
The effect of the infection in the pig is minimal, as the parasite does not seem to penetrate the mucosal surface. In humans and monkeys, the parasite can enter the surface of the large intestine causing extensive tissue erosion. Furthermore, the parasite may penetrate through the muscularis mucosae into the submucosa and spread out radially causing rapid destruction of the tissues. Most of the lesions caused by Balantidium coli are found in the caecal and sigmoid rectal regions.
The symptoms vary from severe diarrhoea and dysentery to mild insignificant, almost asymptomatic condition.
Epidemiology
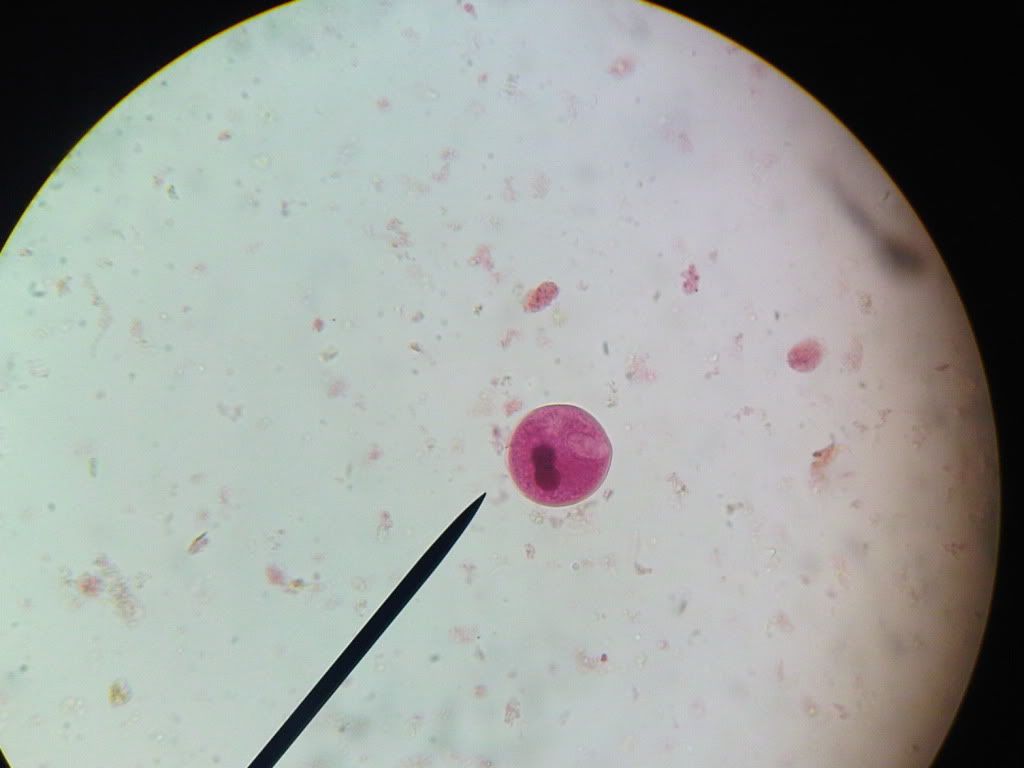
Infection is acquired by the ingestion of cysts of Balantidium in food or water contaminated with faecal matter. Since balantidiasis is common in dogs and primates, it may be expected that these animals are probably the main source of infection to man. However, human infections are usually associated with people who come in contact with pigs, chiefly pig keepers or butchers.
The infection rate is very low in humans and this is because humans are generally refractory to exposure to Balantidium. However, once the infection has established itself in man, there is usually a rapid transmission from one person to another, particularly where there is no proper sanitation.
Diagnosis and treatment
Slide smears of infected faeces should show characteristic cysts or trophozoites of Balantidium coli. The infection responds to treatment with tetracycline, and metronidazole.
Leishmaniasis
Leishmaniasis
These haemoflagellates of the genus Leishmania are parasites of invertebrates and vertebrates that use small biting sand flies (Phlebotomus), such as P. sargenti, P. papatasii, P. argentipes and many others, depending on the geographical area as vectors. The leishmanias are rounded or oval parasites with a morphologically complex life cycle consisting of a promastigote with a free flagellum and amastigote that has no flagellum.
Human leishmaniasis is scattered over wide areas of the globe, from China Asia to India Iran Afghanistan Near East , the Mediterranean basin, into Sudan Ethiopia West Africa . In the New World , it extends fromMexico Argentina
The diseases attributed to Leishmania range from a mildly inconvenient lesion of the skin, known as Oriental sore, to a serious disease involving the liver and spleen, known as kala-azar. In South America , one form of leishmaniasis (mucocutaneous) extensively involves the mucous membranes of the mouth, nose and pharynx, as well as the skin but does not affect the viscera.
Leishmaniasis is a zoonotic disease that is maintained in nature by many animals including dogs, rodents, wolves, foxes, jackals, raccoons, sloths and marsupials. Numerous species of Leishmania have been described in mammals and of these three are generally accepted as human parasites; two of these cause cutaneous leishmaniasis and the third causes visceral leishmaniasis or kala-azar.
Life cycle, symptomatology and epidemiology
The amastigote forms of Leishmania are ingested by sand flies when they feed on the blood and tissue fluid of vertebrates. Compared with trypanosomes, development of Leishmania in the sand fly seems quite simple, involving the transformation of the amastigote forms to promastigotes. These promastigotes multiply in the digestive canal of the insect and transform into infective promastigotes which are injected into a new vertebrate host when the fly feeds again.

Life cycle
In the vertebrate the promastigotes enter host macrophage cells, roundup and lose their flagella, forming the so-called Leishman bodies or amastigote forms that are the characteristic forms found in the vertebrate. The parasites multiply and increase within the host cells until they cause them to rupture. The rapture of the host cells liberates more parasites that are engulfed by other phagocytic cells.
Table. Leishmania species and main characteristics associated with infections
Species
|
Characteristics
|
Major foci
|
L. aethiopica
|
Cutaneous, self-healing, also diffuse, zoonotic
| |
L. donovani
|
Visceral, kala-azar, anthroponotic
| |
L. infantum
|
Visceral, zoonotic (dogs)
| |
L. major
|
Cutaneous, self-limiting, rural, zoonotic
|
Mediterranean basin and
|
L. tropica
|
Cutaneous, self-healing, with possible recurring satellite lesions, urban, anthroponotic
|
Mediterranean basin and
|
L. braziliensiscomplex
|
Cutaneous and mucocutaneous, zoonotic
| |
L. chagasi
|
Visceral, zoonotic (dogs)
| |
L. mexicanacomplex
|
Cutaneous, and a few mucocutaneous reported in
| |
Cutaneous leishmaniasis (Oriental Sore)
Cutaneous Leishmaniasis is characterised by sores on the face, arms and legs especially in children. The aetiological agent isLeishmania tropica, a parasite that has a wide distribution in Asia , Middle East and Africa .
The classical lesion of cutaneous leishmaniasis is the Oriental Sore, which is about 5 to 15 cm in diameter and takes several months to develop following infection. Incubation period is usually between 2 to 7 months or longer. The infection is usually accompanied by spontaneous healing of the sore after 9 to 12 months, sometimes leaving unsightly scars on the skin. There is increasing evidence that the infection is followed by permanent immunity.
The sores do not usually contain pus except when they have been invaded by bacteria. Classical sores are found from the Mediterranean region to India Asia and parts of Africa . Another form of Oriental sore is the relatively wet one that attacks the lymph nodes. This has a shorter incubation period than the dry Oriental sore. It is probably a zoonosis since it occurs in rural areas where its reservoirs are dogs, ground squirrels and gerbils.
Mucocutaneous leishmaniasis (Espundia)
Mucocutaneous leishmaniasis is a chronic disease that occurs in Central and South America . The aetiological agent isLeishmania braziliensis and the most severe form of the disease is called espundia which is endemic in the jungles of South America , particularly Peru Bolivia Brazil

Oriental sore (A and B) and espundia (C)
Visceral Leishmaniasis (kala-azar)
Visceral leishmaniasis is caused by L. donovani, a parasite similar to both L. tropica and L. braziliensis. Visceral leishmaniasis exists in China India Afghanistan Sudan Ethiopia East Africa , South and Central America . It attacks the reticuloendothelial cells of the spleen, liver, bone marrow and visceral lymphatic nodes. This is a rural disease with dogs, foxes, rodents and other mammals acting as its reservoirs.
Biting sandflies introduce promastigotes into the dermal region of the skin. The promastigotes enter the blood and lymph vessels and make their way to the liver, spleen, and bone marrow via lymph and blood. As the parasites divide and increase in numbers, they cause the rupture of the macrophages and release of more parasites that become available to infect more macrophages. The destruction of the macrophages continues until their numbers are considerably reduced.
To counteract the loss of macrophages, the bone marrow is activated to produce more macrophages at the expense of other important cells such as polymorphonuclear cells. The resulting monocytosis and leucopoenia weaken the defence system of the body very badly. The result is that many of the victims of kala-azar succumb to infections that they would normally resist.
Clinical kala-azar develops when the liver, spleen, kidney, and bone marrow are infected. Very often kala-azar is fatal if not treated. In other people, however, the symptoms may assume a chronic course.
The symptoms of kala-azar include headaches, irregular bouts of fever with a temperature (which can range from 36 oC to 40 oC) and oedema of the face, trunk and feet. Other symptoms include substantial weight loss, splenomegaly, hepatomegaly, and anaemia, bleeding from the gums, lips, nose and intestinal mucosa.
Leishmania donovani exhibits geographical host-relationships. In China India
In the Mediterranean region where dogs are important reservoirs, children are usually more affected than the adults. In theSudan Sudan Americas Sudan Nile grass rat), Acomys albigena (the spiny mouse), the genet and the serval. In the semi - arid northern districts of Kenya Uganda
Diagnosis
In cutaneous leishmaniasis, the presence of parasites can be demonstrated in blood smears made from skin scrapings, aspiration of juice from the wound or biopsy. Alternatively, the scrapings from the lesion can be cultured or inoculated into hamsters and examined for the presence of amastigotes. In kala-azar, a biopsy from the bone marrow or spleen can be examined for the presence of amastigotes, cultured on a suitable media or inoculated into hamsters before being examined. In either case, the presence of amastigotes confirms the infection.
Treatment
Proper parasitological identification of the parasites is essential for correct treatment. This is because the treatment of leishmaniasis is long and the drugs are toxic. Cutaneous leishmaniasis is usually self-limiting and most of the wounds heal within 6-9 months. The infection can therefore be left to take its course until it heals, unless the wounds are disfiguring because of bacterial involvement. In many parts of southwest Asia , children are exposed to infection or given live virulent injections of the parasite in order to induce immunity that will protect them against further infection and thus avoid disfiguring lesions and scars on the face.


A case of kala-azar, with enlarged liver and spleen

Mucocutaneous leishmaniasis and kala-azar are more serious than cutaneous leishmaniasis because they are not only incapacitating they can also be fatal. The first line of treatment involves the administration of pentavalent antimonials, such as stibogluconate, or meglumine antimoniate. The second line drugs if the antimonials fail are amphoterin B and pentamidine. If untreated, kala-azar has a mortality rate of 100 %.
Vector Control
The diversity of the epidemiology of the different forms of the disease makes it impossible to control leishmaniasis with a single approach or tool. Infection can be prevented by use of repellents or insecticides against sandflies. The elimination of dogs and other reservoir hosts greatly reduces the risk of infection. Vector control using suitable insecticides are effective against peridomestic transmission.

Saturday, January 19, 2013
Phylum Aschelminthes
Phylum Aschelminthes
Six different classes, dominated by the Class Nematoda, represent Aschelminthes as a phylum. The nematodes (Nema, thread) or roundworms are ubiquitous small worms that dwarf in numbers all other groups of multicellular animals except insects and mites.
Aschelminthes are pseudocoelomate, unsegmented worms that are covered with a cuticle. An unusual aschelminth trait is parthenogenesis (development of an unfertilised egg). In the classes Nematoda, Rotifera and Gastrotricha, males may be lacking and successive generations of females are produced parthenogenetically
Still another remarkable characteristic of the phylum is the consistency of cell numbers (eutely), in which a precise and relatively small number of cells, remaining unvaried throughout the animal’s life, comprise both specific organs and the entire animal. The number, constant not only for the species but also for the taxonomic group, can be used as part of its morphological definition (for example, the central nervous system in Ascaris and many other nematodes consists of 162 cells)
Class Nematoda
The class Nematoda is very important to us among the group as it consists of parasites of medical and veterinary importance. The class is characterized by the presence of a complete digestive system, consisting of a mouth, intestine, anus and absence of cilia.
Nematodes are found practically in every ecological setting. They live in animals and plants as parasites and in mud, marine, fresh and brackish waters, soil and mud as free-living organisms. They vary in size from a few microns to slightly over a meter in length.
Morphology
Nematodes are cylindrical in shape, tapering at both ends of the body. The digestive system is a long tube that runs from the buccal cavity to the anus. In hookworms, the mouth is armed with teeth or cutting plates. Some nematodes are provided with lips, papillae or a leaf crown around the external opening of the mouth. The pharynx (oesophagus) is a strong muscular tube with walls that can contract and expand, creating a pump-like suction mechanism. The neuromuscular system consists of a circumesophageal ring or nerve ring around the oesophagus and dorsal and ventral nerve cords, which give rise to motor and sensory nerves. The excretory organs lie within the lateral lines and open via a pore ventral to the oesophagus.
Internal structure of a nematode
The gonads lie in the pseudocoele, a space between the intestine and the body wall that is filled with a fluid. The rugged cuticle is not only an extraordinary effective protection against harmful external substances but also serves as an exoskeleton to which muscles are attached.
Female nematodes have a pair of ovaries consisting of cells (oogonia) that produce the eggs. The uteri are usually packed with eggs. The eggshell material is produced by the uterine cells. The uteri unite to form the vagina, which may contain a seminal vesicle for storing male sperm. The vagina opens to the outside by a pore or vulva at the middle of the body, though it may also be near either end of the body.
The male genital system consists of one testis and a seminal vesicle, which continues posteriorly as a muscular ejaculatory duct that opens into the rectum or cloaca. The cloaca serves as a conduit for sperm and digestive wastes. Copulatory spicules protrude through the cloaca opening. The spicules, usually two in number, unite and form a tube through which spermatozoa are injected into the vulva and vagina of the female.
Generalized structure of male (A) and female (B) nematode
Nematodes are classified into two subclasses: Phasmidia (have caudal sensory organs) and Aphasmidia (lack caudal sensory organs). The phasmids include most soil nematodes, most parasites of insects and vertebrates. The aphasmids are mainly aquatic forms and a few parasitic ones.
Life cycle
Life cycles differ greatly among the many nematodes that are parasites of man. Mature female worms produce eggs, which pass out of the body with faeces. Most of the eggs are produced when they are not embryonated and become embryonated in the soil. The embryonated eggs are ingested and under the action of the gastric juices of the host, the larvae are liberated from the eggs. In some parasites, such as Ascaris, the larvae penetrate the wall of the small intestine and migrate to the lungs via the blood. From the lungs, they reach the pharynx and finally settle in the small intestine, where they will attain maturity, mate and produce eggs.
In other nematodes, such as hookworms, the larvae hatch from eggs in the soil and after going through several moults become infective. These third stage infective forms enter the body through the skin. Trichinella has a unique life cycle in nematodes in that the entire life cycle is spent in the host. Transmission from one host to another is through predation, cannibalism or carrion feeding.
Microscopic Techniques
Microscopic Techniques
Most parasitic agents occur within the gastrointestinal region, body tissues and blood system. Most helminth parasites (nematodes, trematodes, cestodes) and some protozoa inhabit the intestine as well as the bile and pancreatic ducts that empty into the intestinal lumen. The helminths produce characteristic eggs, which pass out in faeces that are used for identification. Either the protozoa exist as active trophozoites or as inactive cysts and their presence in a stool sample is an indication of an infection.
Some important stains for blood films
Method for cleaning microscope slides
Slides used in laboratories for identification purposes should be clean from dust and grease. Films do not stick well if the slides are dirty and greasy and stained artefacts may give the wrong results. There are several ways of cleaning blood slides but the most practical method is the one that uses Decon
Make up a 2 – 3 % solution of Decon in hot water.
Place slides into the solution individually.
Allow slides to stand in solution for at least ½ hour (1 – 2 hours is better).
Rinse thoroughly in running tap water for an hour.
Rinse briefly in distilled water.
Store slides in ethanol – dry as required or dry and store in clean boxes.
Giemsa’s Stain
This stain can be used in different dilutions. A one in ten dilution is convenient, as it gives rapid staining, although the time varies according to the quality of the stain.
The stain can be used for thin films and thick films.
Method for the thin films
Pour 2 ml of Giemsa into a measuring cylinder and make up to 20 ml with distilled water. Empty the dilute stain into a staining jar.
Fix the blood film by immersing it in methyl alcohol for 2 – 3 minutes.
Wash the slide containing the film in distilled water.
Drain off the water and transfer the slide into a staining jar containing Giemsa.
Stain for 30 – 60 minutes.
Remove the slide and rapidly wash it in distilled water.
Wipe off stain from the back of the slide and leave it in an upright position to dry.
Examine in immersion oil at 100 % magnification.
Method for thick films
Prepare the final stain as for the thin film.
Without any fixation, place the slide in the stain.
Leave for 15 – 30 minutes in the stain (by agitating the staining jar gently from time to time, haemoglobin is removed from the area of the film).
Remove the slide from the stain and wash in distilled water.
Let it dry
Leishman’s Stain
Method for thin films
Place the slide on a staining rack over sink or dish, with the film facing uppermost.
Cover the film with 12 drops of the stain and leave for 1½ minutes (the methyl alcohol in the stain fixes the film).
Add double the quantity of freshly distilled water.
Mix the solution thoroughly with a pipette or by rocking very gently the slide, taking care not to spill the stain.
Allow to stain for 15 to 20 min, the actual time depending on the quality of the stain. The film should be quite a strong pink colour, when sufficiently stained. The colour is then reduced or the film differentiated by washing with distilled water.
Flood the slide with distilled water, then tip and allow the water to run off. Continue to wash with distilled water until the film is a rather pale pink.
Stand the slide in an upright position to drain and dry. Heat must not be used to dry films but the film can be gently dabbed with blotting paper and waved in the air to expedite drying.
Examine in immersion oil at 100% magnification.
Method for thick films
Thick films must be treated very gently at all stages, as they are not firmly attached to the slides.
Place the slide film side downwards in a dish (or stand it upright in ajar) containing tap or distilled water.
Leave until all the haemoglobin has been removed (2 to 3 min) and the film has become completely white.
Stand the slide in an upright position to drain and dry.
Proceed as for staining thin films.
Field’s stain for thick films
Field’s stain is used for rapid diagnostic and identification purposes. For thin films, fix in methyl alcohol for 2 to 3 minutes prior to staining.
Preparation
A. Methylene blue (medicinal) 0.4 g
Azur 0.25 g
Buffered water (B), pH, and 6.8 – 7.0. 250 ml
B. Disodium hydrogen phosphate 10.0g
Potassium dihydrogen phosphate 12.5 g
Distilled water 1000 ml
C. Eosin 0.5 g
Buffered water (B) 250 ml
Method
Dip slide in A 1- 2 sec
Rinse in B 2- 3 sec
Dip in C 1- 3 sec
Rinse gently in tap water 2- 3 sec
Place slide upwards to drain and dry
Examination of Stool for Intestinal Protozoa
Direct Method
Place a fresh sample of stool, about the size of a bean seed, on a slide, add a drop or two of physiological saline, cover with a cover slip, and examine at a magnification 400 - 500X. This technique should reveal the motile forms of intestinal flagellates.
To identify the cysts and the nuclei of Amoeba, one or two drops of a 4% Lugo
Examination of Stool for Worm Eggs
a) Direct Examination
A sample of faeces, about the size of a bean seed, is placed on a slide, mixed with tap water or physiological saline, covered with a cover slip, and examined at low microscope magnification (100 to 200X).
The smear should not have lumps of faeces to obscure the eggs. A drop of Lugo
b) Thick Smear Technique (Kato and Miura)
Preparation of the Kato stain
Distilled water 500 ml
Glycerine 500 ml
Malachite green, 3% in water 5 ml
Place about 100 mg of faeces on a slide and cover it with a cellophane strip (size 26 x 28 mm), which had been soaked in the Koto Stain for at least 24 hr. Press over the strip with a spatula to have a thin uniform spread of the faeces sample. Allow the preparation to stay at room temperature for ½ to 1 hr. Examine the stool. The glycerine clears the faeces and helminth eggs can be seen at a magnification of 100x.
c) Concentration Method
Place 1 g of stool in a test tube and thoroughly mix it with a concentrated salt solution. Remove any plant particles floating on the surface and allow the mixture to remain undisturbed for 20 to 30 min.
Helminth eggs float on the surface because they are lighter than the salt solution, Transfer the eggs to a microscope slide using a wire loop. Examine at a magnification of 100-200x.
d) Universal Concentration Method
Place 1 g of faeces in a small beaker, add 7 ml of 50% hydrochloric acid and the same amount of ether. Mix the mixture to form a homogeneous solution. Pass the mixture into a centrifuge tube over two layers of muslin placed in a funnel. Centrifuge the solution for a minute or two.
Four layers are formed: the uppermost layer is a yellowish zone of ether below which is a zone consisting of food particles and below this is a zone of hydrochloric acid. The bottommost zone consists of small particles and worm eggs. Carefully insert a pipette into the bottom layer, suck out liquid, and examine it for eggs. Be careful, ether is very volatile and can explode!
Sedimentation Concentration Stool Examination Technique
Add an estimated 5 g of stool to 100 ml of formol glycerol solution (5 ml formaldehyde, 10 ml glycerol, 985 ml of water) in a flask and mix with a glass rod.
Sieve through a mesh into another flask.
Add more formol glycerol up to 3 cm below the top and allow to sediment for a minimum of 20 min at room temperature.
Pour off the supernatant to leave approximately 25 to 30 ml of fluid containing sedimented deposit
Resuspend in formol glycerol and sediment for 20 minutes.
Gently pour off supernatant to leave 15 to 20 ml of suspended deposit.
Use straw to transfer about 0.1 ml of the deposit to each of 3 microscope slides and cover each slide with a cover slip.
Examine
Subscribe to:
Posts (Atom)




